Many reactions like oxidation, halogenation, nitration, Grignard, pH adjustment as part of work-up and product isolation etc can be very fast reactions in which the reaction rate is faster or comparable with the mixing rate. Typically, fast reactions are associated with competitive side reactions and hence scale-up of these reactions needs to be based on detailed mixing analysis. Fast competitive reactions are part of synthesis route of many pharmaceutical active ingredients and specialty products and are critical from scale-up and overall economy of the synthesis process.
For Right First Time Scale-up of fast competitive reactions. a number of parameters needs to be considered like stirrer type – axial or radial or mixed flow or any other type of impeller, agitation speed, feed point location, single or multiple feed points, and dosed reagent concentration. The typical challenges encountered in the scale-up of fast competitive reactions are:
- Local concentration near addition point may increase leading to higher impurity formation
- Inhomogeneity in the turbulent energy distribution rate: f = eFP / evol avg changes between scales
- Macromixing or blend time increases
- Mixing regime may change from micromixing at lab scale to mesomixing at plant scale
This application note explains use of CFD based mixing simulation software MixIT for agitator design and selection of plant reactor for specific process- in this case, scale-up of a fast oxidation reaction. The reaction involves oxidation of alcohol to aldehyde using sodium hypochlorite and a phase transfer catalyst – having risk of over oxidation from aldehyde to acid which will result in considerable yield loss.
Reaction scheme:
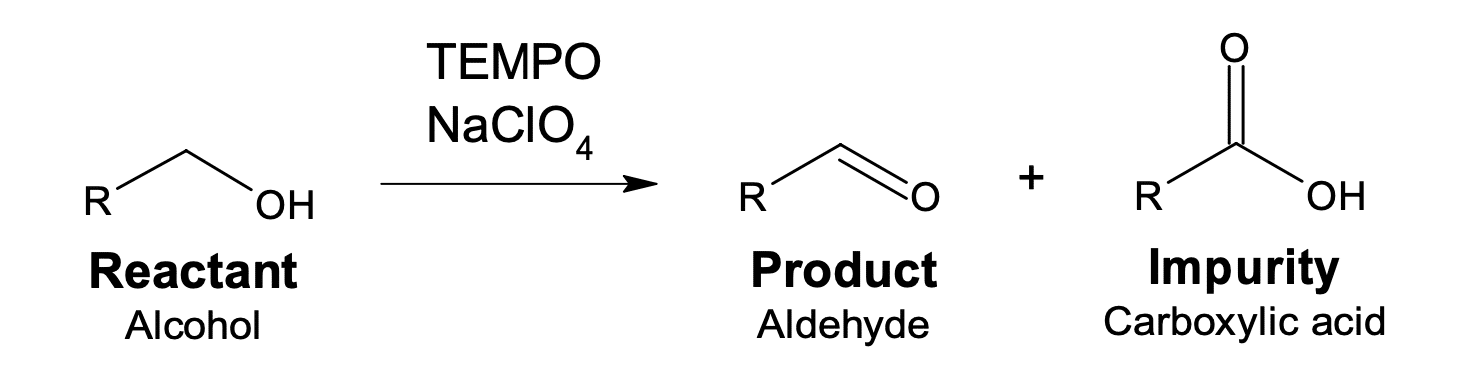
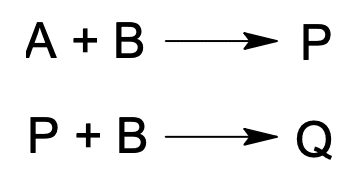
The reactions of product and impurity formation are represented by reactions in series as:
Laboratory scale studies:
The reaction was optimized in the laboratory scale. Three parameters were identified which resulted in higher acid impurity formation-
Longer hypochlorite addition time (over 40 min): Longer addition time of Hypochlorite resulted in higher acid impurity formation. As the concentration of intended product Aldehyde increases in the reaction mass, the probability of product molecules reacting with oxidizing reagent being added in to reaction mass to form acid impurity increases. A lowest addition time will favour higher yield of Aldehyde.
Longer mixing time (over 40 min): A longer mixing of biphasic reaction mass post fast addition of oxidizing agent resulted in higher acid impurity formation.
Higher temperature above 15°C: Reaction temperature above 15°C resulted in higher acid impurity formation due to kinetics effects.
Fast addition of hypochlorite solution within optimized temperature range followed by switching off of the agitation enabled aqueous-organic layer separation producing highest yields of desired product aldehyde and prevented formation of acid impurity and hence yield loss. A total residence time more than 40 min resulted in increase in acid impurity.
Scale-up Studies:
Addition time of hypochlorite solution in plant scale was limited to a minimum of 20 min due to exothermic nature of the reaction. However, even addition in 20-25 min can lead to over-reaction due to higher mixing time scales in the plant reactor and fast addition requires similar or timescales at micro, meso and macro levels. In addition, droplet size needs to be comparable to ensure similar mass transfer for the liquid-liquid heterogeneous reaction process. Three plant agitator configurations were compared with the lab scale golden batch to select best fit to replicate lab scale mixing environment during scale-up.
Simulation results:
CFD simulations were run to compare lab and plant scale mixing. Further, tracer simulations were run to calculate macromixing or blending time. The lab scale study was conducted in a 2L cylindrical reactor having 1L reaction mass and fitted with PBTD agitator and 4 baffles. Three different agitator configurations were evaluated for 6000L plant scale reactor with 4000L reaction mass: Single PBTD, a two stage PBTD and an anchor – all with four baffles. The reagent dosing was simulated using dip tube for PBTD agitators and surface addition for anchor.
The tracer simulation is compared in figure 1 and the results are compared in table 1 below. As seen in the figure, blending with anchor agitator is faster and close to lab scale reactor where as PBTD agitator gave longer blending time even with two stages.
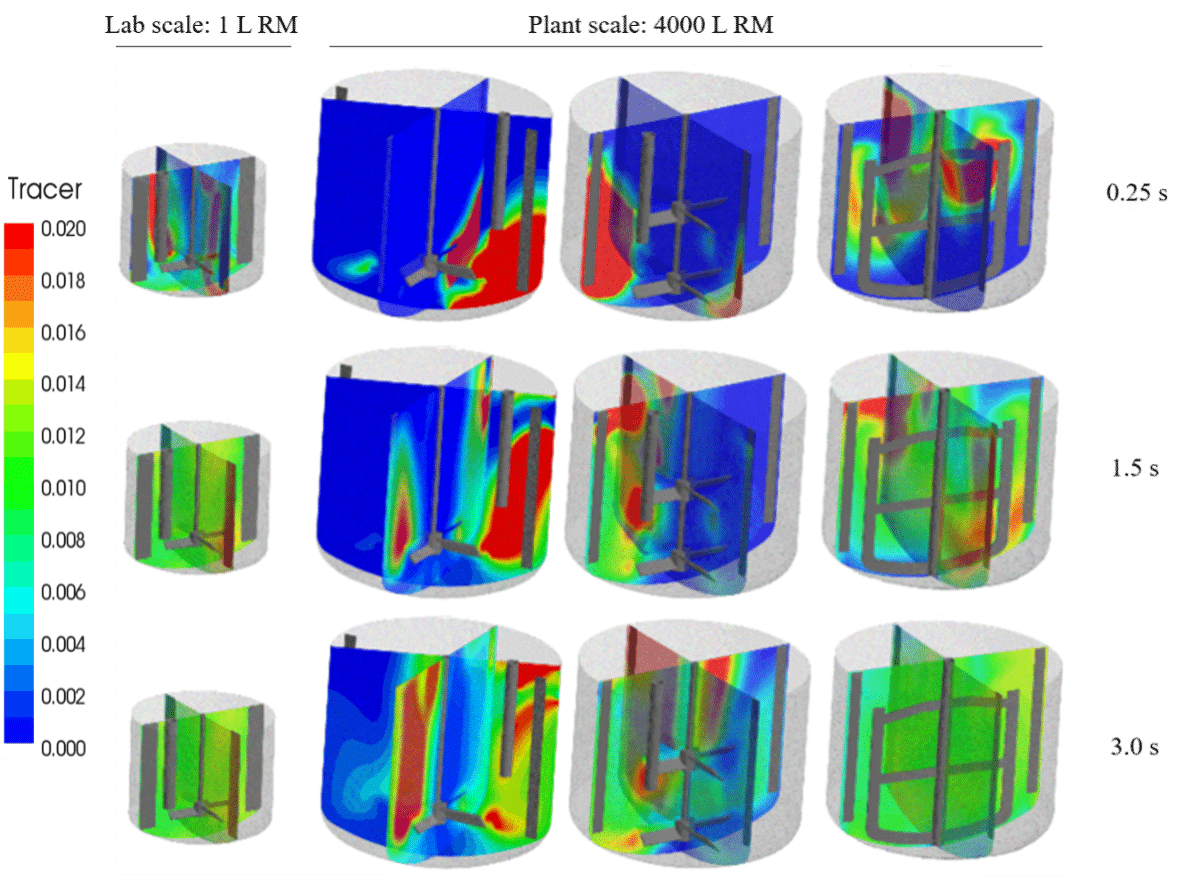
Figure 1: Comparison of lab and plant agitation: Tracer concentration at different timelines
In comparison with lab scale, power per unit volume (P/V) is lower for single PBTD, comparable for two stage PBTD and more than double for anchor agitator. Clearly, in this case, constant P/V is not the scale-up parameter. For fast competitive reactions, micro, meso and blending time need to be comparable. The micromixing time in plant scale was comparable or lower in comparison with lab scale. Mesomixing time for all plant agitators was higher than lab scale for 10min reagent dosing time. The plant anchor agitator was the closest match with the lab scale with 20 min dosing time in terms of mesomixing and macromixing time and with lower micromixing time and was selected for scale-up validation.
Table 1: Comparison of mixing parameters for lab and plant scale
| Mixing parameter | Lab Scale: 1L RM | Plant scale: 4000 L RM | |||||
| Single PBTD | Two PBTD | Anchor | |||||
| RPM | 550 | 100 | 100 | 56 | |||
| P/V, kW/m3 | 1.06 | 0.74 | 1.23 | 2.37 | |||
| Micromixing time, s | 0.0117 | 0.0138 | 0.0108 | 0.0076 | |||
| Mesomixing time, s | 0.124 | 0.233 | 0.181 | 0.134 | |||
| Blending time, s | 1.7 | 13.7 | 9.18 | 4.85 | |||
| Droplet size, mm | 293 | 312 | 264 | 188 | |||
| Mesomixing time, s | Addition time (s) | Addition time (min) | |||||
| 5 | 10 | 20 | 10 | 20 | 10 | 20 | |
| 0.124 | 0.293 | 0.233 | 0.23 | 0.181 | 0.169 | 0.134 | |
Conclusion:
The fast competitive oxidation reaction was successfully scaled-up from 1L to 4000L scale based on sound understanding of different mixing parameters calculated from CFD and tracer simulation. The simulation allowed selection of best fit plant scale agitator that with lab scale. A decision based on constant P/V would have resulted in a wrong scale-up strategy in this case study of fast competitive reaction and may have resulted in higher impurity and lower yield upon scale-up.









Leave A Comment