Over 80% of the intermediates and products in the Specialty Chemical and Pharmaceutical Industry are Solids. The unit operation that produces a product in a solid form and removes impurities is “Crystallization”.

Crystallization is a critical unit operation that strongly influences product quality, yield, and downstream performance. Yet, it is also one of the most failure-prone steps during scale-up. Many challenges observed in filtration, drying, purity, and batch consistency originate from improper mixing.
Ensuring the same mixing environment across lab, pilot, and plant scales is essential for achieving right-first-time crystallization. This blog explores why crystallization scale-up fails, the role of mixing, and how MixIT enables predictive, CFD-based crystallization scale-up, supported by real industrial case studies.
Crystallization Challenges Are Often Mixing Problems
Poor crystallization process introduces challenges across entire down-stream processing, including:
- Batch-to-batch variability
- Poor stirrability and filtration
- Yield and purity losses
- Polymorphic contamination
- Excessive washing and product loss
- Long drying times and residual solvent issues
- Fines generation, agglomeration, and rework
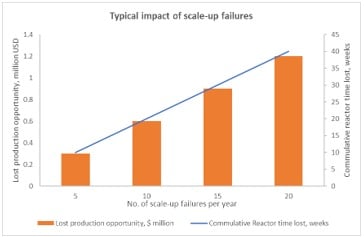
These issues significantly increase manufacturing costs and time to market. In most cases, they can be traced back to improper mixing during crystallization scale-up.
Why Mixing Plays a Central Role in Crystallization Scale-Up
Mixing directly impacts nucleation, growth, and crystal breakage. During scale-up, changes in agitation, reactor geometry, and liquid height alter local hydrodynamics, leading to different crystallization behavior.
Competitive reactions reduce yield by consuming valuable starting materials, and the products of these side reactions can accumulate as impurities that may be difficult to remove from the final product. These impurities may alter the crystal structure of some products, resulting in undesired polymorphic crystal forms.
An improper mixing at scale can result in off spec failure, longer filtration and drying times, yield losses due to need of recrystallization and hence impact profit margins.
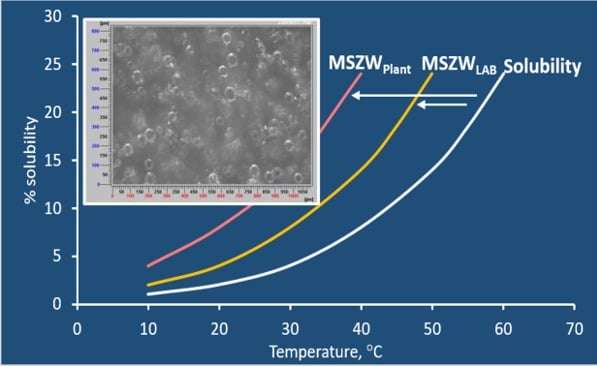
Mixing has an impact on MSZW (metastable zone width) and hence nucleation point from lab to plant scale can vary resulting in different product attributes at plant scale.
Improper mixing can result in:
1. Agglomeration or oiling out.
2. Settling of seeds, making seed ineffective.
3. Locally high supersaturation at the antisolvent addition point causing pre-matured nucleation and fine Particle Size Distribution
4. Highly vigorous mixing may cause particle breakage or attrition causing fines generation
Antisolvent crystallization- Locally high supersaturation:
In antisolvent crystallization, supersaturation is generated by increasing the antisolvent-to-solvent ratio, which results in a reduction in solubility as represented by the solubility curve.
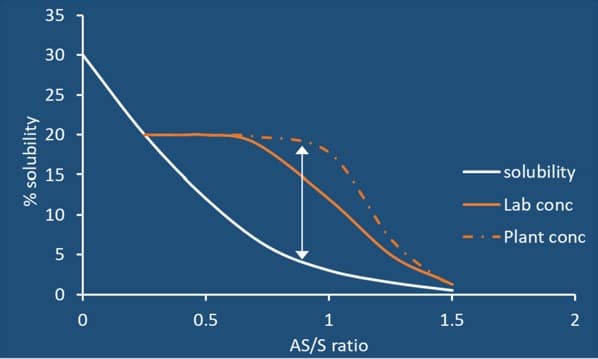
During antisolvent addition, the concentration profile within the reactor becomes critical, particularly in the region near the point of addition. If the antisolvent is not dispersed sufficiently fast, localized zones of very high supersaturation are created, leading to a meso-mixing effect. Under these conditions, the effective nucleation point, or metastable zone width (MSZW), shifts locally compared to laboratory conditions. As a result, premature nucleation takes place in the antisolvent addition region, resulting in higher nucleation rates and fine particle size distribution compared to laboratory batches. This can lead to slow filtration rates, slow drying times, overall increased batch time cycle (productivity loss) and batch failure in product quality.
At larger scales, these localized supersaturation regions promote excessive nucleation relative to crystal growth, resulting in fine particle generation. While laboratory-scale experiments may produce larger, well-formed crystals that are easy to filter and dry, insufficient mixing during scale-up can lead to a significant reduction in crystal size and a broader particle size distribution, creating downstream filtration and drying challenges.
Reactive Crystallization – Locally High Supersaturation
In reactive crystallization, supersaturation is generated through a chemical reaction, typically by the addition of a counter-ion to form a sparingly soluble salt. In this process, the reaction occurs first, followed immediately by precipitation; however, salt formation reactions are generally very fast, making the system highly mixing-sensitive.
The reactants are usually much more soluble and present at higher concentrations compared to the solubility of the product salt, which results in the rapid generation of very high local supersaturation and extremely fast precipitation kinetics. Under these conditions, reaction and precipitation occur simultaneously, and the particle characteristics are governed by the interplay between precipitation and reaction kinetics, fluid mixing across micro-, meso-, and macro-mixing scales, and turbulence and shear rates.
Local concentration gradients arising from inadequate mixing strongly influence nucleation and growth, as well as aggregation and particle break-up. From a scale-up perspective, maintaining control over the hydrodynamic state, particulate phase evolution, and local concentrations is particularly challenging, and these interactions make it difficult to simultaneously achieve the desired polymorphic form and particle size distribution at production scale.
MixIT: A day-saver for Crystallization Unit Operation!
MixIT has a global track record of enabling process engineers/R&D chemists to aid with its 3D CFD analysis and get a visualization of what exactly is happening in the reactor. What exactly needs improvement in mixing to get a Smart Right-First-Time Scale-up.
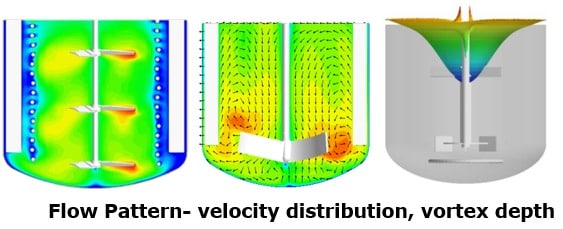
Provide ‘a-priori’ prediction of various hydrodynamic parameters:
- Flow patterns
- Energy dissipation rates
- Mass transfer rates
- Vortex depth
- Strain rate distribution
- Mixing profiles
- Solids distribution profiles
These profiles allow identification of flow patterns, including whether single or multiple circulation loops are formed, as well as regions of high velocity, low velocity, and dead zones. The tool also predicts energy dissipation rate distribution, vortex depth, and shear and strain rate distributions, which can vary significantly between lab, pilot, and production scales. Such differences directly impact particle size distribution when the shear environment is not similar across scales.
Meso-mixing time can be evaluated to determine whether it is a controlling parameter in the process, as poor meso-mixing can lead to locally high supersaturation and premature nucleation. This information can be used to identify optimal reagent addition locations, preferably in regions with lower meso-mixing timescales. In addition, MixIT enables analysis of solid suspension behavior, including estimation of the just-suspended speed (NJS), and the ratio of actual operating speed to NJS, helping ensure uniform solid dispersion and consistent crystallization performance across scales.
Case Study 1: Reproducing Bulk Density During Site-to-Site Transfer
Challenge
A two-step crystallization process (cooling followed by anti-solvent addition) needed to be transferred from a client site to a CDMO. While the client achieved the desired bulk density, the CDMO observed lower bulk density at lab and pilot scale.
MixIT Insight
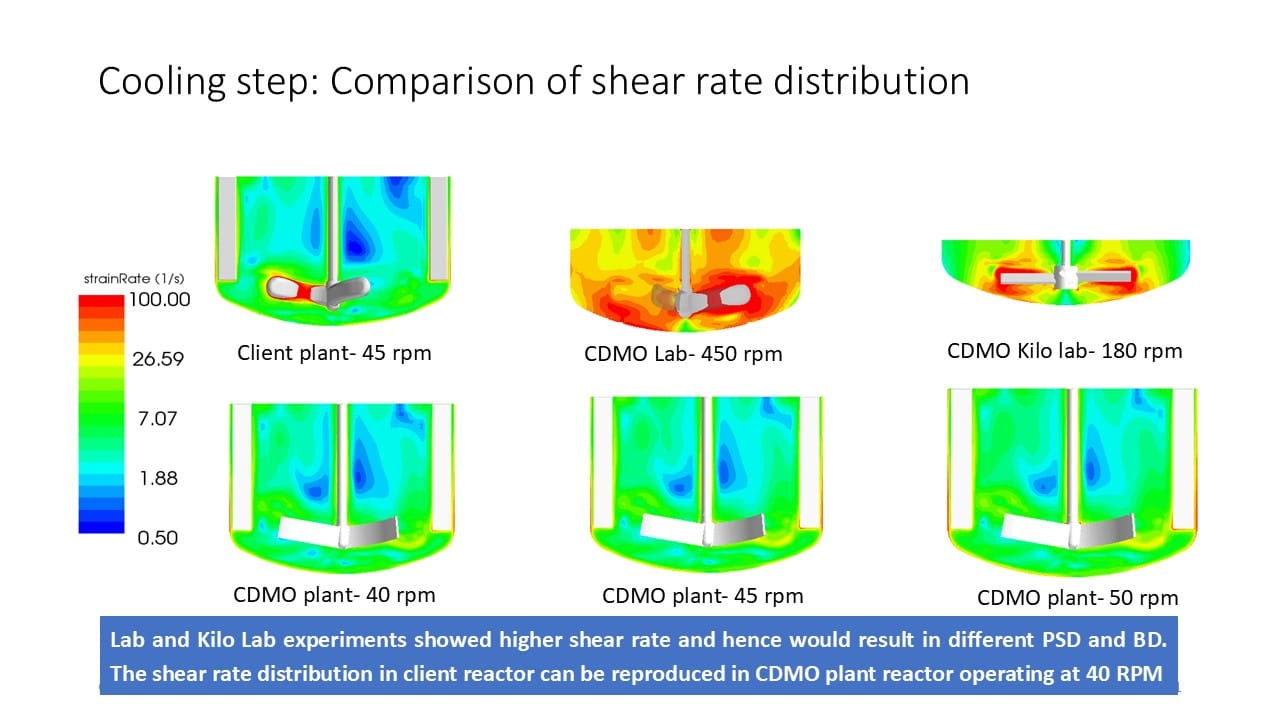
CFD simulations revealed higher shear rates in the CDMO lab and kilo-lab reactors compared to the client reactor. By matching the shear rate distribution, MixIT identified the optimal RPM range for the CDMO plant reactor.
Outcome
- Cooling crystallization optimized at 40–45 RPM
- Anti-solvent addition optimized at 45–50 RPM
- Equivalent mixing environments achieved
- Bulk density successfully reproduced at the CDMO site
This enabled confident, right-first-time site transfer without repeated experimentation
Case Study 2: Quick CAPA for a Crystallization Batch Failure
Challenge
An anti-solvent crystallization batch failed PSD specifications due to very fine particles. A rapid CAPA was required to continue plant validation under ICH Q10 guidelines.
MixIT Insight
By comparing micro-mixing times between passed lab batches and the failed plant batch, MixIT identified RPM as the key control parameter affecting nucleation intensity.
Outcome
- Failed batch successfully reworked at reduced RPM
- CAPA completed within 2 days using simulations
- Avoided at least 2 weeks of lost reactor time
- Enabled confident scale-up for subsequent validation batches
Business Impact of MixIT-Led Crystallization Design
MixIT is recommended by our customers from Top Speciality Chemical and Pharma Manufacturing Companies for:
- Predictable crystallization scale-up
- Reduced development and validation risk
- Improved yield, PSD, and product quality
- Faster site-to-site transfer
- Lower operational and rework costs
Most importantly, it shifted decision-making from empirical trial-and-error to science-based, predictive process design.
Conclusion
Crystallization scale-up failures are rarely accidental—they are often the result of poorly understood mixing environments. MixIT’s 3D CFD-based mixing analysis provides the insight needed to design, scale, and troubleshoot crystallization processes with confidence.
By enabling a priori decisions and hydrodynamic matching across scales, MixIT helps organizations save money, reduce risk, and achieve right-first-time crystallization.
Request a MixIT Demo
If crystallization challenges are impacting your product quality, yield, or timelines, MixIT can help you predict, optimize, and scale your process with confidence.
Request your personalized MixIT demo here: https://mixing-solution.com/register-for-a-free-trial/







Leave A Comment